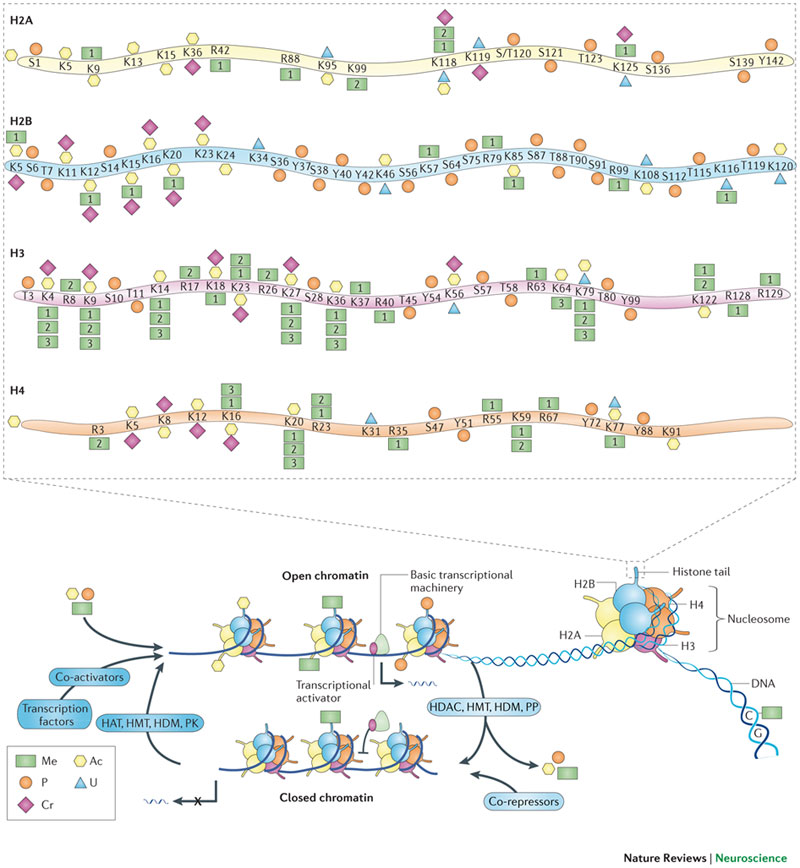
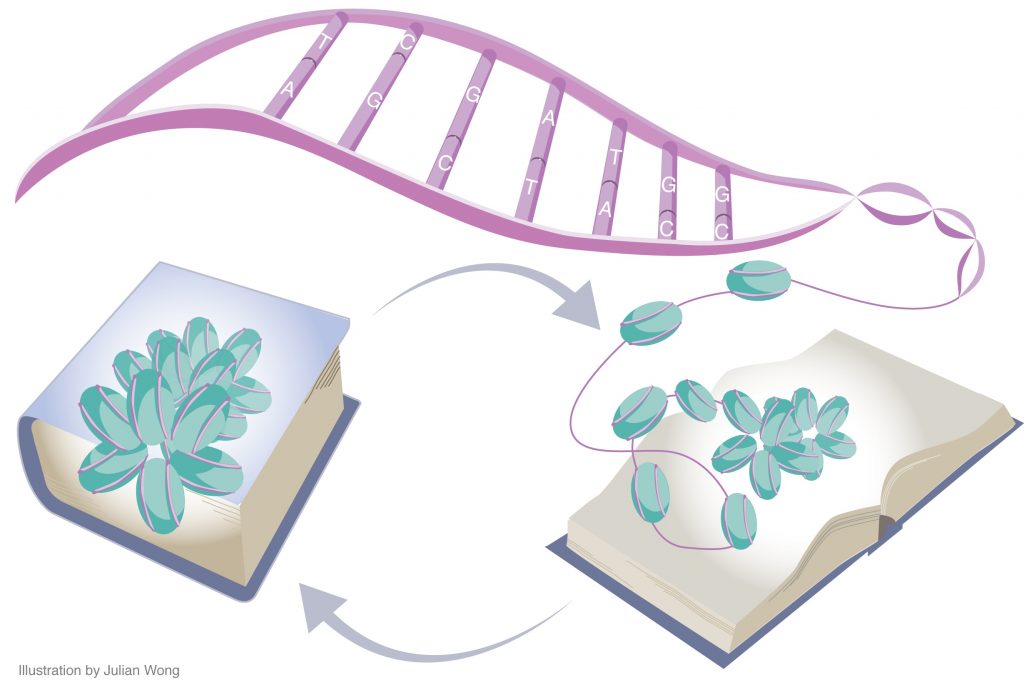
Long-lasting memories require specific gene expression programmes that are, in part, orchestrated by epigenetic mechanisms. Of the epigenetic modifications identified in cognitive processes, histone acetylation has spurred considerable interest. Whereas increments in histone acetylation have consistently been shown to favour learning and memory, a lack thereof has been causally implicated in cognitive impairments in neurodevelopmental disorders, neurodegeneration and ageing. As histone acetylation and cognitive functions can be pharmacologically restored by histone deacetylase inhibitors, this epigenetic modification might constitute a molecular memory aid on the chromatin and, by extension, a new template for therapeutic interventions against cognitive frailty.